Allocetra™: off-the-shelf cell therapy designed to restore macrophage homeostasis.
Allogeneic mononuclear cells collected from healthy donors and induced to a stable apoptotic state.
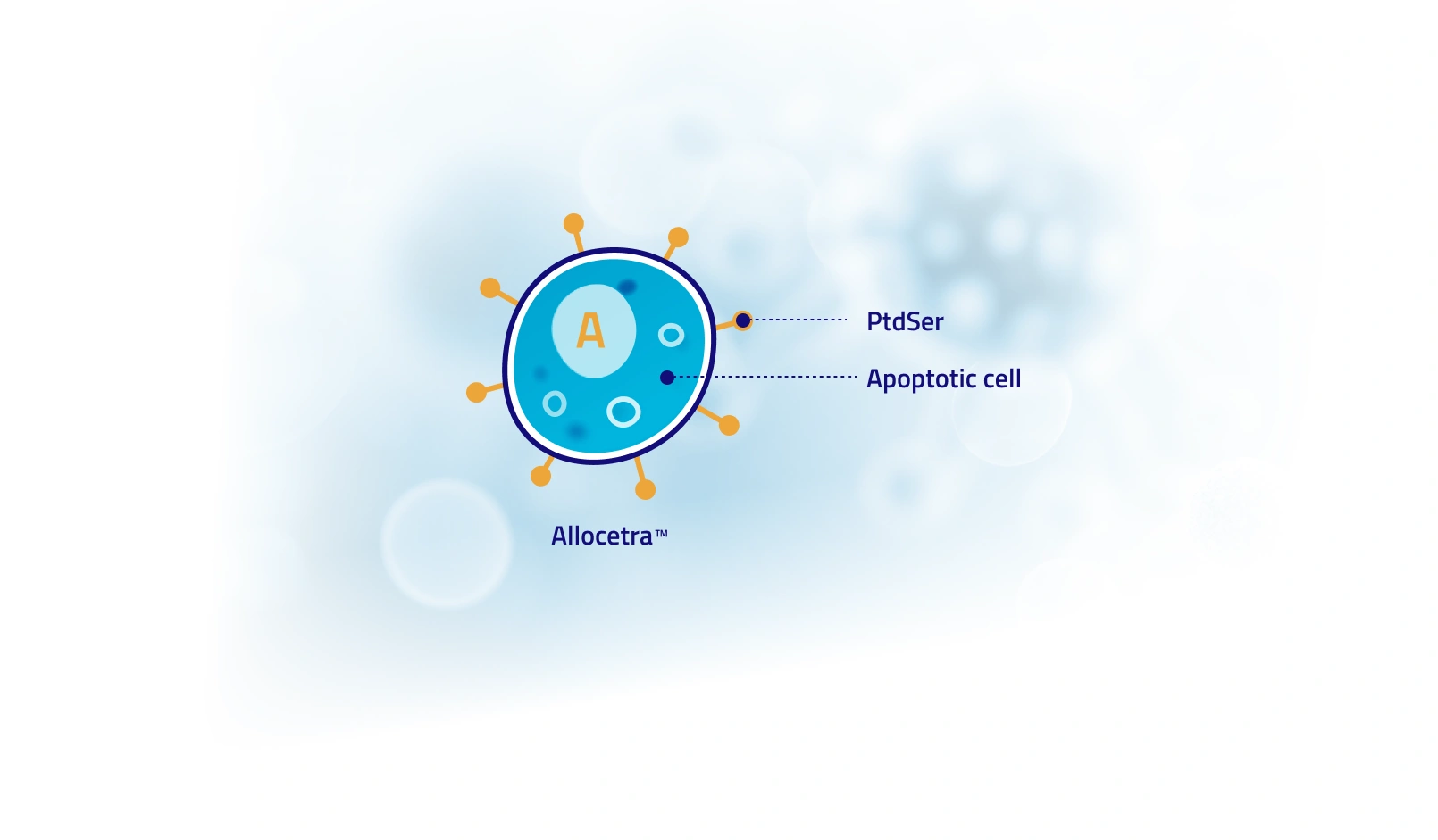
Allocetra™ is an off-the-shelf, cell-based therapy composed of mononuclear cells derived from non-HLA matched donors and induced to an apoptotic state. It is designed to be administered as a stand-alone treatment or in combination with leading therapeutic agents.
Representing a paradigm shift in macrophage modulation, Allocetra™ shifts the focus from targeting specific subsets or pathways to a fundamental approach centered on restoring macrophage homeostasis. By rebalancing the immune system and returning it to normal levels of operation, Allocetra™ promotes disease resolution and has the potential to provide a novel immunotherapeutic mechanism of action for patients with debilitating and life-threatening clinical indications.
Novel therapeutic modality:
macrophage modulation.
Validated approach:
harnesses natural processes of apoptotic cells.
Clinically tested:
favorable safety profile across 150+ patients.
Cost-effective cell therapy:
highly differentiated, off-the-shelf cellular therapy.
Over 150 patients have been treated with Allocetra™ infusion in clinical trials, with no reported serious adverse events or safety signals attributed to the treatment. The accumulated data indicate a favorable benefit-risk ratio, supporting the further evaluation of Allocetra™ in clinical trials.
Enlivex is conducting several clinical studies in osteoarthritis, which is a degenerative disease with low grade inflammation, and an indication with a substantial unmet medical need, that potentially represents a multi-billion commercial market.
Our Mode of Action
Administration of Allocetra™ introduces apoptotic cells into the target environment, triggering the modulation of macrophages, restoring their homeostatic state, and effectively rebalancing the immune system.
This unique therapeutic approach to induce macrophage homeostasis begins with the infusion of Allocetra™ into the patient. Through well-defined mechanisms, the Allocetra™ cells interact with the patient’s macrophages, restoring their homeostatic state. Using this inherent immune pathway, Allocetra™ can shape a patient’s innate immune response.
Allocetra™ naturally expresses phosphatidylserine (PtdSer), a primary “eat me” signal of apoptotic cells, harnessing their naturally occurring activity to induce pro-homeostatic effects on macrophages and other antigen presenting cells (APCs). Apoptosis, a normal and critical process in tissue homeostasis, results in the swift removal of dying cells by APCs capable of apoptotic cell engulfment, such as macrophages and dendritic cells. Exposure to apoptotic cells induces several changes in the engulfing APCs, generally promoting an anti-inflammatory response at the tissue level, as well as immunological tolerance.
Overactive macrophage
Allocetra™ with PtdSer signaling “eat me” signal to macrophage
Engulfment
Homeostatic macrophage
Allocetra™ is derived from cells collected from the peripheral blood of healthy unrelated donors.
Through our proprietary manufacturing process, the cells are induced to a stable apoptotic state and resemble a naturally occurring biological mechanism. Allocetra™ doses are formulated and cryogenically preserved, ready for clinical use.
As an allogeneic cell therapy, with a streamlined manufacturing process, Allocetra™ presents a cost-effective solution for treating acute and chronic inflammatory conditions, offering a competitive pharmacoeconomic model.
VIEW OUR PIPELINEOur Manufacturing Process
Collect cells from healthy donors
Our Manufacturing Process
Apoptosis induction and
stabilization process
Our Manufacturing Process
Cells express ‘eat me’ signal
Our Manufacturing Process
Cells are frozen
Our Manufacturing Process
Off the shelf cell therapy
How it works
Allocetra™ offers flexible administration options, including systemic intravenous infusion or targeted local (intra-articular) injections, depending on the specific indication and medical need.
Formulated and packaged for immediate clinical use, Allocetra™ enables healthcare professionals to quickly and easily administer the therapy to patients without the need for manipulation, dilution, or mixing.
1 Patient with systemic or joint imflamation
-
2 Allocetra cells are injected into the patient
-
3 Allocetra cells are engulfed by macrophages
-
4 Macrophage homeostasis is restored
